
Most emulsions contain droplets with a mean diameter of >1µm, however mini-emulsions and nano-emulsions can be formed with droplet sizes in the 100-500 nm range, and with proper formulation, highly stable microemulsions can be prepared having droplets as small as a few nanometers. These emulsion types are shown schematically in Figure 1. A triple emulsion with water dispersed in oil droplets, with themselves then dispersed in water, is referred to as a water-in-oil-in-water emulsion (w/o/w) and the less common reverse analogue is termed oil-in-water-inoil (o/w/o). More complex “triple” emulsions have dispersed droplets that contain smaller droplets of continuous phase material. Emulsifiers reduce the energy required to break the dispersed phase into droplets and prevent them from coalescing by generating a repulsive force or a physical barrier between them.Įmulsions that are formed by dispersing a water-immiscible liquid into an aqueous phase are termed oil-inwater (o/w), while those having aqueous droplets dispersed in a continuous oil phase are termed water-in-oil (w/o). Emulsions also contain emulsifiers – materials that concentrate at the phase interface to lower the interfacial tension.

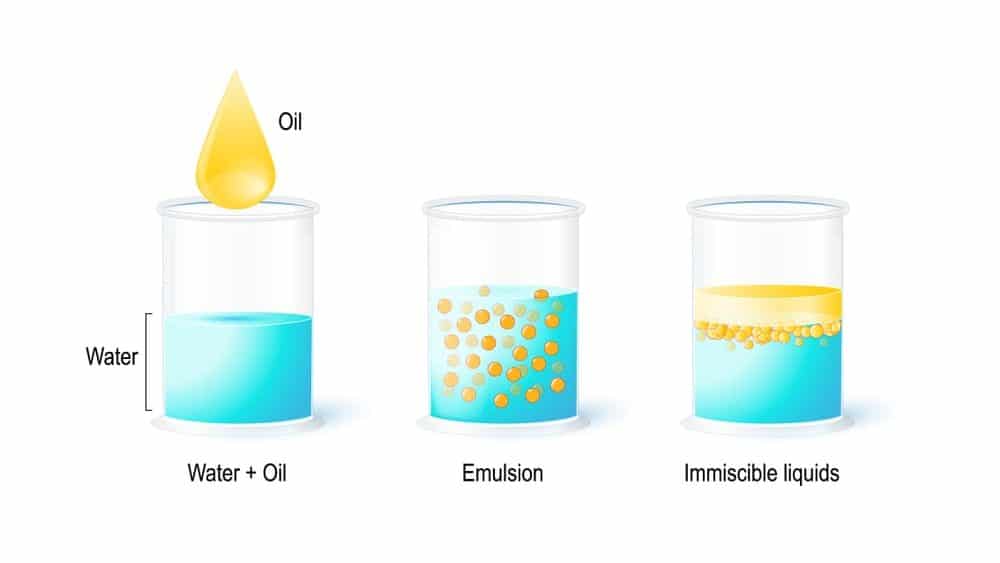
In its simplest form, an emulsion is a mixture of two immiscible liquids (usually an organic “oil” and water) in which one liquid (the dispersed phase) is in the form of microscopic droplets dispersed in the other (continuous) phase.

Emulsions are also used as precursors to prepare polymer microparticles, solid lipid nanoparticles, inorganic nanoparticles and oilfilled microcapsules and have been developed as precursors to magnetic particles for imaging, diagnostics and drug delivery. Implantable Drug Delivery Systems and Combination Products Dosage FormsĮmulsions are used in a wide variety of industrial and pharmaceutical products including ocular, topical, mucosal, intravenous, intramuscular, and oral products.Solubility & Bioavailability Enhancement.


 0 kommentar(er)
0 kommentar(er)
