
Working with elements from aluminum (which has an atomic number thirteen) to gold (seventy nine), he was able to show that the frequency of these transitions increased with each element studied. Moseley was able to confirm these two hypotheses through experimentation, measuring the wavelengths of photon transitions of various elements while they were inside an x-ray tube. The number of protons in an atom is referred to as the atomic. The Bohr model of the atom had the central charge contained in its core, with its electrons circulating it in orbit, much like how the planet in the solar system orbit the sun. For example carbon atoms have six protons, hydrogen atoms have one, and oxygen atoms have eight. Two years later, Henry Moseley and Niels Bohr made further contributions that helped to confirm this. Antonius van den Broek added to this by formerly suggesting that the central charge and number of electrons were equal. This central charge would be roughly equal to half of the atoms total atomic weight. It was he who first suggested the model for an atom where the majority of its mass and positive charge was contained in a core. The atomic mass is typically listed in the periodic table below the.

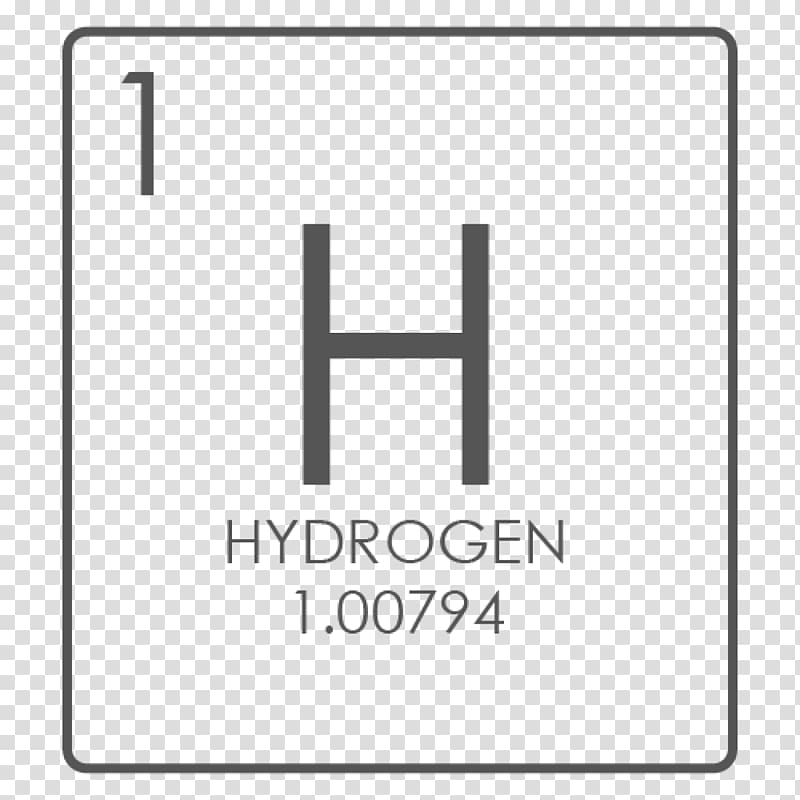
The atomic number of an element never changes, meaning that the number of protons in the nucleus of every atom in an element is always the same.Īrranging elements based on their atomic weight began with Ernest Rutherford in 1911. The atomic mass of Hydrogen is 1.00797 amu and the atomic mass of Carbon is 12.011 amu. Oxygen atoms contain 8 protons and have an atomic number of 8, and so on. All carbon atoms contain six protons and therefore have an atomic number of 6. For example, Hydrogen atoms, which have one proton in their nucleuses, are given an atomic number of one. The mass numbers of hydrogen’s isotopes are 1, 2, and 3, the most abundant being the mass 1 isotope generally called hydrogen (symbol H, or 1 H) but also known as protium. You will need to refer to a periodic table for proton values.Ever wonder why the periodic table of elements is organized the way it is? Why, for example, does Hydrogen come first? And just what are these numbers that are used to sort them all? They are known as the element’s atomic number, and in the periodic table of elements, the atomic number of an element is the same as the number of protons contained within its nucleus. In this notation, the atomic number is not included. The elements are arranged in increasing order of atomic number. The atomic mass is the weighted average of the isotopes of the element. Let’s get back to the atomic mass on the periodic table. However, there seems to be a discrepancy. Symbol-mass format for the above atom would be written as Cr-52. For example, the atomic number of hydrogen is 1, so it is represented as H1. The atomic mass listed for hydrogen on the period table is 1.00794 amu.
For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The "A" value is written as a superscript while the "Z" value is written as a subscript.

Both the atomic number and mass are written to the left of the chemical symbol. The composition of any atom can be illustrated with a shorthand notation called A/Z format.


 0 kommentar(er)
0 kommentar(er)
